Clean Room Validation Ppt
Dr Farshid SADEGHIPOUR EAHP Foundation Seminar Patient Safety. In case that an ECA is used for the cGMP manufacturing process the HVAC and cleanroom validation is a mandatory requirement of each environmental controlled area as established by the Federal Register.

Clean Room Design And Qualification Ppt Download
ISO 14644 A cleanroom consists or either a single room or a number of interconnected rooms where the concentration of airborne and work surface particles are known and limited to pre-defined levels in addition to the control of related environmental factors such as viable and non-viable particles temperature air pressure airflow.

Clean room validation ppt. We will later consider individual components in detail. Clean Room Validation Definition Clean Room A room in which the concentration of airborne particles is controlled and which is constructed and used in a manner to minimize the introduction generation and retention of particles inside the. Of course a well-designed air handling system must not only be properly designed but also properly installed qualified and maintained sealed ducts tight filters. Must be designed properly by professionals. Validation protocols are a method of establishing documented evidence that shows a high degree of assurance that a manufacturing process will consistently yield a product of repeatable high quality. HVAC and Clean Room Validation.
FS 209E is concerned about the following particle sizes in microns. Cleanroom validation is more than simply counting particles. Cleanroom man and materials entry from adjacent. Basics of Clean Room. A cleanroom is a modular environment in which the following environmental factors are kept under control. 01 02 03 05 50.
To ensure that the facility equipment and environment meets User Requirement Specifications URS. In line with GMP guidance we provide Design Qualification DQ Installation Qualification IQ Operational. Validation has several phases beginning with design qualification and ending in final certification. To ensure that the design of the facility is fit for its intended purpose. To ensure that the design of the facility is fit for its intended purpose. Principles of Cleanroom Validation.
Cleanroom validation is provided by conducting a series of tests to qualify if a controlled environment is performing in accordance with process requirements and the applicable regulatory guidelines such as ISO 14644-12015 or GMP Annex 1. Choose a chair for what it can do to make your employees job healthier and more comfortable. Cleanroom ValidationHVAC Validation is performed for a variety of reasons. The initial setup of a cleanroom requires a validation to be performed over a specified period of time to ensure that the cleanroom is function- ing as required over the given period of time. Cleanroom Validation Procedures Preliminary Considerations Purpose of Cleanroom. Over this period of time historical data are collected to ensure that.
HVAC Heating Ventilation Air Conditioning validation continues to be a source of anxiety for many manufacturers of pharmaceutical biologic products. Provides Clean Room Classes Rooms classified based on number of particles 05 micron per cubic foot Class descriptions still in use today. VALIDATION OF CLEAN ROOMS FOR ASEPTIC MANUFACTURING FRSmal Pharmaceutical Director ICCE ICCE a member of SNC-Lavalin Group tel 3226431600 E-Mail. Cleanroom Furnitures in Singapore - Utopia. With a CAGR of 5 from 2017 to 2025. Must be treated as a critical system.
More About Compounding 23-25 May 2008 Krakow Poland Cleaning validation of clean-rooms and preparation equipments 12 Non-toxic to operators Non-flammable Fast-drying but not reasonably so Not harmful to clean room surfaces Not likely to leave particles or residue that. Temperature airborne particulates microbes relative humidity differential pressure and air flow. GMP Requirement All facilities and machinery are correct for the purpose and that they and the environment in which they are situated is properly cleaned and appropriately treated. VALIDATION OF CLEAN ROOMS Proving that the environmental conditions of the clean rooms that have been defined in the HVAC ORDER. SRP ENVIRO SERVICES PVT. Cleanrooms are designed to maintain extremely low levels of particulates such as chemical va PowerPoint PPT presentation free to view.
Introductory Validation and the Cleanroom - These regulations do not. It includes numerous different tests that must be performed in various cleanroom states in order to verify that the cleanroom is fit for its intended use and meets the stipulations set forth for the classification requirements governing the cleanroom application. QTS guides you in the right direction. Global Cleanroom technology Market - Global Cleanroom Technology Market size is projected to be valued 52 Billion by 2024. Biofit Clean Room Chairs is the integral part of Work Place ergonomics. To ensure that the facility equipment and environment meets User Requirement Specifications URS.
FOUR BASIC PRINCIPLES OF CLEAN ROOM Not To Bring Any Dust Not To Accumulate Any Dust Not To Generate Any Dust To Remove Any Dust Quickly 8 9. To ensure that the facility equipment and environment meet defined regulatory requirements. To ensure that the design of the facility is fit for its intended purpose. Four basic requirements of cGMP are safety identity strength and purity which can be achieved by cleaning process and its proper validation Clean room Validation is performed for a variety of reasons. INTRODUCTION Air handling systems Play a major role in the quality of pharmaceuticals. The HVAC and cleanroom validation is performed every time an Environmental Control Area ECA is required and used for GMP manufacturing purposes.
This entails a thorough inspection and several tests whereafter the cleanroom is certified to a specific class indicating its level of control usually to an ISO14544-1 class. To ensure that the facility equipment. Types of Clean Room. Some of the components particularly the filters are essential to ensure the quality of the air. Validation studies should demonstrate that Class 100 is maintained in critical zones during routine operations. Cleanroom facilities are designed to go from lower class to higher class less clean to more clean Each subsequent clean space requires additional controls to prevent ingress of undesired items Goal is to make it more difficult for contamination to occur as you get cleaner Minimize critical area space.
HVAC validation is likely to be successful when good strategy and planning are involved in the process. Sterile Area Cleanroom Qualification Sterile area validation has different tests like air supply air velocity air changes flow pattern filter integrity pressure test particle count temperature recovery test microbial count relative humidity noise level and vibration test. Cleanroom Validation is performed for a variety of reasons. Cleanroom Type PowerPoint PPT Presentations. Most often cleanrooms are validated by third-party validation agencies.

Clean Room Design And Qualification Ppt Download

Clean Room Design And Qualification Ppt Download

Cleanroom Classification Design And
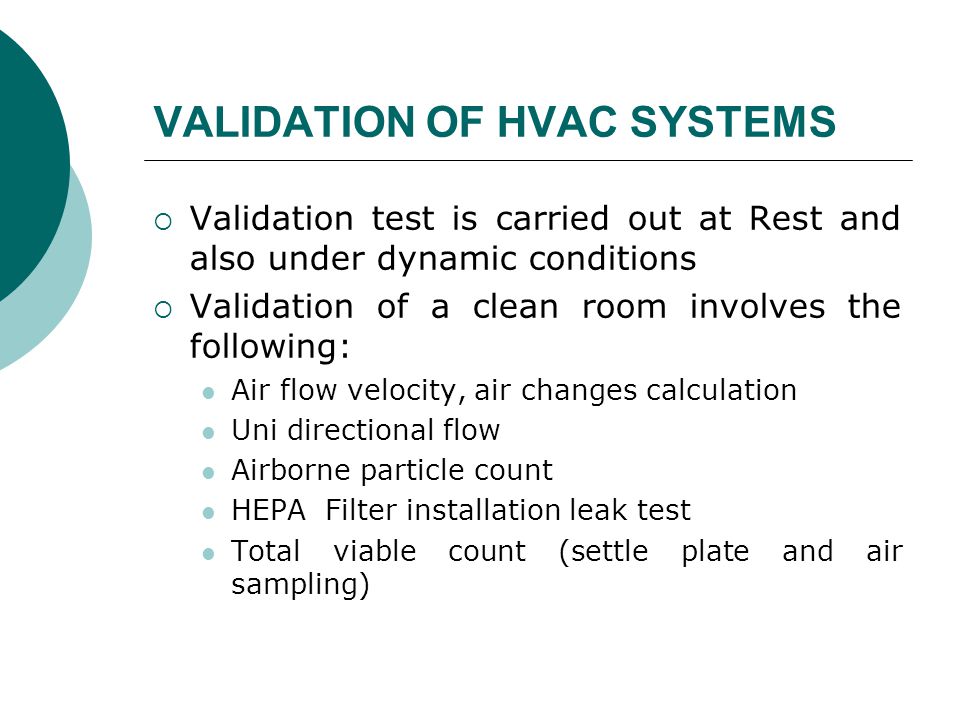
Validation Of Hvac Systems Ppt Video Online Download

Validation Of Hvac Systems Ppt Video Online Download

Ppt Verification And Validation Powerpoint Presentation Free Download Id 3728669

Introduction To Pharmaceutical Clean Room Ppt Video Online Download

Cleanroom Classification Design And

Clean Room Design And Qualification Ppt Download

Ppt The Cleanroom Approach To Quality Software Development Powerpoint Presentation Id 4326061



Posting Komentar untuk "Clean Room Validation Ppt"